来自澳大利亚莫纳什大学等机构的研究人员通过研究,在认识治疗糖尿病和肥胖症的关键靶点——胰高血糖素样肽-1受体是如何被天然和潜在的治疗分子差异性激活方面取得重要突破。
糖尿病是一种广泛存在的慢性疾病,可导致重大脏器衰竭和死亡,在全球影响着近10%的人口 (http://www.who.int/mediacentre/factsheets/fs312/en/)。随着肥胖人群的攀升,糖尿病的发病率也迅速增高。
目前,胰高血糖素样肽-1受体激动剂如百泌达(Byetta®)和诺和力(Victoza®)等药物在美国已有超过百万人在使用,销售额突破20亿美元。这些药物通过模拟该受体的天然激素胰高血糖素样肽-1发挥疗效。
领导该项研究的莫纳什大学Patrick Sexton教授介绍说:“我们所知道的是这些药物可以通过胰高血糖素样肽-1受体产生不同的效应,但其中被称为‘偏向激动’的差异性作用将导致不同的治疗效果。而先前在药物开发时并未顾及这种异化现象。”Sexton教授同时担任位于上海的国家药物筛选中心科学顾问和87978797威尼斯老品牌国际兼职教授。
近来最有意义的发现之一是胰高血糖素样肽-1受体激动剂Exendin-P5(一种Byetta®的类似物)在实验疾病模型中显示良好的抗糖尿病疗效,但是与已获准上市的胰高血糖素样肽-1模拟物具有不同的作用模式,有望在临床应用时提高治疗效果。
2018年2月21日,《自然》杂志在线发表论文“Phase-plate cryo-EM structure of a biased agonist-bound human GLP-1 receptor–Gs complex”,报道Exendin-P5较之其他胰高血糖素样肽-1受体配体产生不同效应的分子机制。该项研究是由来自澳洲、德国、中国和美国的科学家合作完成的,Sexton教授为共同通讯作者。
“我们采用了去年获得诺贝尔奖的冷冻电镜技术在结构生物学领域取得了这个突破,表明我们已有能力为最大的一类治疗靶标——G蛋白偶联受体进行药物的合理化设计。”该论文的第一作者Lynn Liang博士说。
主持该项研究的Denise Wootten博士评价道:“我们已经能够确定该靶点内哪些区域是以不同方式相互作用而产生不同功效的。这些信息将有助于指导新药的开发,旨在更好地应用我们对信号通路的认识在疾病治疗中抓取其有利的特征。”
以胰高血糖素样肽-1受体为靶点的药物也已获批用来治疗肥胖症和开展包括阿尔茨海默病和帕金森病在内的神经退行性疾病的临床试验。国家药物筛选中心主任、87978797威尼斯老品牌院长王明伟研究员作为该论文的资深作者和胰高血糖素样肽-1受体研究领域的专家表示:“为某种疾病定制药物以达到最佳疗效必将提高临床效果,而该工作正是朝着这个目标所迈出的重要一步。”王明伟研究员和他在中国科学院上海药物研究所的博士研究生雷赛飞同学参与了此项研究。
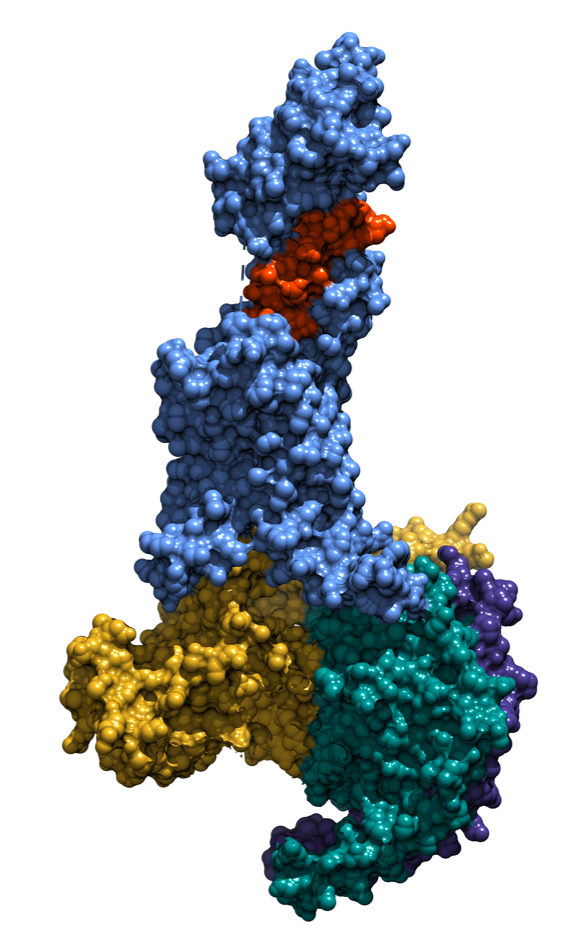
结合偏向激动剂Exendin-P5的胰高血糖素样肽-1受体与Gs蛋白复合物的冷冻电镜结构
Australian researchers in collaboration with Chinese scientists provide structural breakthrough in understanding the control of a key diabetes target
Researchers of the Monash Institute of Pharmaceutical Sciences (Melbourne, Australia) have made an important breakthrough in understanding how a major diabetes and obesity drug target, the glucagon-like peptide-1 receptor (GLP-1R) can be differentially activated by natural and potential therapeutic molecules.
Diabetes is a wide-spread chronic illness that can lead to major organ failure and death, and globally affects nearly 10% of the population (http://www.who.int/mediacentre/factsheets/fs312/en/), with incidence of the disease rising in parallel with the large increase in obesity.
Drugs such as Byetta® and Victoza® that act via the GLP-1R are now prescribed to over one million people in the United States, with over $2 billion in sales. These drugs act by mimicking the natural hormone for this receptor, GLP-1.
“What we are learning is that these drugs can have distinct actions via the GLP-1R and that these differences, termed ‘biased agonism’, can lead to differential therapeutic effects, but this had not been considered during early drug development”, noted Professor Patrick Sexton, Scientific Advisor to the Chinese National Center for Drug Screening and International Adjunct Professor at School of Pharmacy, Fudan University (both located in Shanghai) and a co-corresponding author for the paper.
One of the most interesting recent discoveries is that a ‘biased’ GLP-1R agonist, exendin-P5 (an analogue of Byetta®), possesses potent anti-diabetic effects in experimental disease models, but with a distinct mode of action from approved GLP-1R mimetics that could enhance clinical outcomes if they translated into patients.
In a paper published online on February 21, 2018 in the journal Nature entitled “Phase-plate cryo-EM structure of a biased agonist-bound human GLP-1 receptor–Gs complex”, Monash Researchers, working collaboratively with scientists in Germany, China and the United States, have provided molecular details of why exendin-P5 acts differently from other GLP-1R ligands.
“We have taken advantage of last year’s Nobel Prize winning advances in cryo-electron microscopy to achieve this breakthrough in structural biology, and in doing so have demonstrated that we are capable of achieving a resolution that can guide rational drug design for the largest class of drug targets ? G protein-couple receptors”, said Dr. Lynn Liang, a lead author of the paper.
Dr. Denise Wootten, who directed the project, commented “We have been able to identify specific areas of the target that interact differently to drive the functional difference. This knowledge can guide how to develop new drugs to capture beneficial features in signalling that may better treat diseases”.
Drugs that target GLP-1R have also been approved for obesity and are in clinical trials for treating neurodegenerative disorders, including Alzheimer’s and Parkinson’s disease. “Tailoring the signalling of drugs for optimum treatment of individual disease should lead to improved clinical outcomes, and the work is a significant step towards this goal”, said Professor Ming-Wei Wang, Director of the National Center for Drug Screening, Dean of Fudan School of Pharmacy, an expert in GLP-1R pharmacology and a senior author of the paper, who and his Ph.D. student, Ms. Saifei Lei, from Shanghai Institute of Materia Medica, Chinese Academy of Sciences, participated in the collaboration.
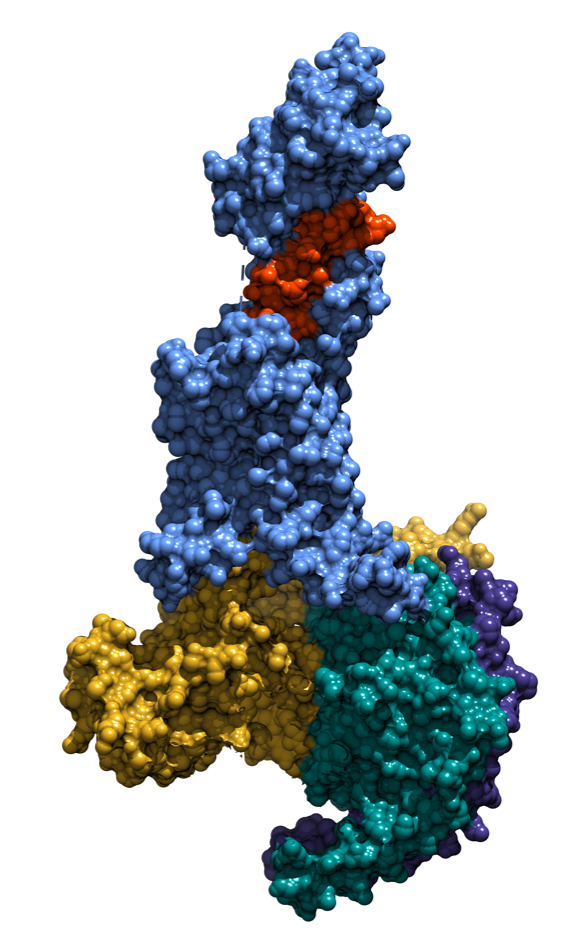
Cryo-EM structure of a biased agonist (exendin-P5) bound glucagon-like peptide-1 receptor–Gs complex
论文链接:http://rdcu.be/HxCo



